
Fondazione Ceschina
Gaetano Ceschina
A Difficult Childhood
The winning idea
The new objectives
In the difficult post-war times Sanitaria was able to manage the transition from a war economy to a peacetime business, turning to innovative products such as rayon and other textile fibers, but also to rubber items and to standard and high-level surgery products. Ceschina acquired in bulk all of the makeshift hospitals created during the war which were about to be dismantled. He then put back onto the market the recovered medical materials. Thereupon he invested the war profits and the accumulated savings in the purchase of buildings. Going beyond Milan he targeted the Adriatic coast, which he had loved ever since his stay in Bologna. That Riviera between Cattolica and Milan was turning into the favorite vacation destination of Milan's gentry. Here he expanded on the land that was already in his possession, devoted himself to building villas and then, in 1926, he built the Dante theatre, so named to honor his firstborn son, which became the center of cultural and artistic activities in Riccione until the Fifties, when it was demolished. In 1928 he built the Grand Hotel in Riccione; one year earlier he had inaugurated the Grand Hotel in Cesenatico. And he also gifted Riccione with its stadium. He broadened his activity in Rimini and even to as far as the opposite shore of the Adriatic sea in Lussinpiccolo, on the island of Lussino, in Croatia, where he renovated hotels and villas. Subsequently, in the post-war period, Ceschina's properties in Croatia were confiscated by Tito's government without any indemnity.
One day, in a port on the Tirrenian coast, Ceschina met a group of glass workers and artists from Murano who were preparing to emigrate, disappointed, without work, because the kilns in Venice and Murano had been destroyed by the bombs and it was assumed that the wealthy foreign customers would no longer return to shop there. He convinced the workers to return to Venice and after a difficult beginning got them involved in a successful venture. He acquired businesses, reopened kilns and exhibition galleries, started the manufacture of artistic glass and lamps and thus recreated an art industry that is a pride of Italy.
The "Sanitaria Ceschina" Company
In 1932 Ceschina was awarded the title of Cavaliere del Lavoro (Knight of Labor) and throughout the Thirties he acquired more health-oriented companies and annexed them to Sanitaria. From the Fascist state he got no special favors; quite the contrary, he was one of the few Milan industrialists who did not join the regime. The Second World War caused him to suffer heavy losses. Several buildings in Rimini, Riccione and Milan were destroyed; the plant in Via Menotti was damaged by two fires. At the end of the war two deaths struck close to him. In Muronico his brother Renzo Ermes passed away, and in September 1945 his son Bruno, a passionate mountain-climber, perished while ascending the Campanile Cornici in the Sasso Lungo group. Gaetano called on his other children to take an interest in the company and devoted himself to the reconstruction of post-war Milan with the activities of Sanitaria, thus creating one of the most solid and powerful Italian companies, which he called Sanitaria Ceschina & C. (S.p.A.). Meanwhile his health was gradually deteriorating.
Gaetano Ceschina passed away on 8 December 1960.
IMAGE GALLERY
The Foundation
"The basic science of medicine, and the future of safe and effective patient care, relies on smart people working in laboratories to answer questions about which they are passionate. We seem to have forgotten that lesson. We need to relearn it quickly."(1)
Fondazione Ceschina is a non-profit foundation under Swiss law created on 10 January 2013, with head offices in Lugano. Fondazione Ceschina aims to promote - in Switzerland and abroad research into cures for rare diseases by incentivating scientific and clinical research in that area.
It is subject to monitoring by the Federal Supervision Authority on Foundations of the Federal Department of the Interior in Berne.
Fondazione Ceschina was born to support research on rare diseases, with particular attention to inflammation-based systemic diseases such as ankylosing spondylitis (Bechterew disease) and more generally ailments that pertain to the class of seroÂnegative spondylartropathies.
In this field we are witnessing a paradox. The interest of the major pharmaceutical houses has led to the introduction in recent years of new drugs that have radically changed the clinical practice, improved the quality of life, the characteristics of the phlogystic involvement and the prognosis of treated patients.
This progress was made possible by the pioneering basic immunology studies made in the Eighties which brought to the identification of key pharmacological targets, such as factor-a tumor necrosis, interleukin 1 and interleukin 6. Most biotechnological drugs interfere with the biological function of these molecules, while other promising targets were identifed subsequently. However, the therapies do not break the vicious circle at the root of the chronic inflammatory response, of the tissue damage and of the productive response that determine the manifestations of these diseases. In other words, even the most efficient drugs currently available and, as far as can be understood, those that will be designed and validated in the coming years do not heal the patients, but modify the entity and characteristics of their inflammatory response by stabilising them at a more acceptable level.
The very efficacy of these drugs is a hindrance to the research on the natural history of these ailments, of which we know very little.
From the vantage point of the pharmaceutical companies there are no rational incentives that would spur them into conducting further research into the causes and the molecular mechanisms involved in the damage associated with the disease, once they've defined a paradigm that allows them to produce effective and reimbursable drugs to which the patient will be hooked†for life. Indeed, it is only by fully understanding the etiology and physiopathology of the ailments that therapies will conceivably be designed to radically alter the natural history and ultimately the result in the patients' actual healing process.
That should be the field in which publicly-financed academic research operates: the one that takes upon itself the necessary and ever less sustainable expenses to provide patients with biotechnological drugs and that should therefore be more keenly interested in identifying new ideas and strategies. And yet, paradoxically, public financing covering these issues is increasingly lagging to the point of being nearly non-existent in most European countries. Even research financed by multinational organisms with some noteworthy exceptions - "is also driving science away from discovery": indeed, it clearly privileges research that has an immediate translational indication, i.e. research aimed at the transfer of information generated in basic areas of human ailments, often to the detriment of studies seeking to identify the causes and the relevant mechanisms of diseases.
Fondazione Ceschina wants to enter into this relatively empty space with a view to favoring research propelled by intellectual passion in the area of rare diseases, by supporting the work of academic groups that have a history of excellence in the study of rare diseases carried forward with originality and consistency. This support includes the financing of projects that are competitive on an international level and tackle issues that are crucial within the fields of etiology, of physiopathology and of the pathogenesis of rare diseases on an immunity-mediated basis. It also extends to the organisation of meetings that may favor discussion, networking and interaction between academic groups featuring the most complementary characteristics.
Even though right now the pieces of the mosaic do not match up with one another in a way that makes sense, we are very close to important advancements in the understanding of immunity-mediated rare diseases. The aim of Fondazione Ceschina for the coming years is to accompany scientists and clinicians in the study and in the exciting work that will make it possible to understand why these diseases commence, why the answer is found in certain parts of the anatomy and not in others, what the role of the recognition of microbial components is and what homeostatic mechanisms must be absent in order for the inflammatory response not to end abruptly.
(1) The Lancet. Catastrophic neglect of the basic sciences in medicine. Lancet. 2012 Apr 7;379(9823):1273. doi: 10.1016/S0140-6736(12)60539-X. This editorial describes in some detail a few of the reasons underlying the "catastrophic neglect of basic research" when it comes to the award of financing in biomedical research. The anonymous author closes with the words "The basic science of medicine, and the future of safe and effective patient care, relies on smart people working in laboratories to answer questions about which they are passionate. We seem to have forgotten that lesson. We need to relearn it quickly."
Scientific Coordinator
Angelo A. Manfredi
Angelo A. Manfredi, MD, is associate professor of rheumatology and area coordinator for rheumatology in the Department of Medicine, at Vita Salute San Raffaele University School of Medicine in Milan, Italy. He is also head of the laboratory of autoimmunity and vascular inflammation in the division of Immunology, Transplantation and Infectious Diseases at the San Raffaele Scientific Institute in Milan. He received his medical degree and his degree in Allergy and Clinical Immunology from the University of Milan and completed postdoctoral training in molecular immunology at the University of Minnesota in Saint Paul.
Dr Manfredi holds three international patents on the use of innate immunity molecules as diagnostic and predictive markers or pharmacological targets in inflammatory disease. He is a scientific advisor and grant reviewer for a number of institutions, including the Istituto Superiore di Sanità , the Ministero della Salute, the Arthritis Research Campaign (UK), MRC (U.K.), Welcome Trust (U.K.), the French National Cancer Institute, the Agence nationale de la recherche (ANR, France), the EMBO fellowship organization, Austrian Science Fund (FWF, Austria), the Icelandic Research Fund (IRF, Reykjavík, Iceland), the Food and Health Bureau (Hong Kong), Reumafonds (Dutch Arthritis Association).
Dr Manfredi has authored papers in peer-reviewed journals such as the Annals of Rheumatic Diseases, Arthritis and Rheumatology, Journal of Immunology, Arthritis Research and Therapy, Science, the New England Journal of Medicine, the Annals of Internal Medicine. He is an associate editor for Clinical and Experimental Immunology and serves on the editorial boards of Dataset Papers in Medicine, The Scientific World Journal, Faculty of 1000 (F1000) Research, and Frontiers in Immunology. He is among the first 1000 most-cited Italian scientists in the world.
From 2013 he is the scientific coordinator of the Fondazione Ceschina.
First Five Years
For the first five years of work of the Ceschina Foundation (2014-2019)[1].
Angelo A Manfredi, University of Vita-Salute San Raffaele, Milano
I gladly take the opportunity to revisit, at least along its main strategic lines, the work accomplished by the Foundation. Anniversaries may not have much sense in a reality as dynamic as the one of the commitment in scientific research. Nevertheless, five years of work represent a noteworthy stretch of road. It may be worthwhile to revisit the things that have been done, to check their consistency with the initial design and to ponder, together, on what are today the priorities and challenges that lie ahead.
A commitment to the intellectual passion in the study of rare diseases. The stated object of the Foundation, upon which we began to ponder, is the support of research on rare diseases, with attention to inflammatory systemic diseases such as ankylosing spondylitis (Bechterew disease) and more generally to diseases that belong to the group of the seronegative spondylarthropathies.
 These conditions generally appear in a youthful age, involve the axial skeleton and pelvis and were identified in the 19th century in particular thanks to the work of Bechterew (in the image to the left), Pierre Marie and Strumpell. A crucial role is played both by the genetic substrate in particular at the level of genes of the major hystocompatibility system such as HLA-B*27 and still poorly characterized molecular pathways that cause the inflammation of the entheses, the anatomical structures that anchor sinews and ligaments to the bone surface, which are essential for locomotion. The response to pathogens (intracellular bacteria and viruses) plays a facilitating and - in animal models - necessary role for the development of the disease. The refinement of the imaging methodologies for visualizing early lesions weighing on the osteoarticular system and the availability of therapies capable of blocking inflammatory molecules such as the tumor necrosis factor alpha and more recently the axis that includes the interleukines 17 and 23, have allowed a sizeable improvement in the stratification of patients and in their clinical management.
These conditions generally appear in a youthful age, involve the axial skeleton and pelvis and were identified in the 19th century in particular thanks to the work of Bechterew (in the image to the left), Pierre Marie and Strumpell. A crucial role is played both by the genetic substrate in particular at the level of genes of the major hystocompatibility system such as HLA-B*27 and still poorly characterized molecular pathways that cause the inflammation of the entheses, the anatomical structures that anchor sinews and ligaments to the bone surface, which are essential for locomotion. The response to pathogens (intracellular bacteria and viruses) plays a facilitating and - in animal models - necessary role for the development of the disease. The refinement of the imaging methodologies for visualizing early lesions weighing on the osteoarticular system and the availability of therapies capable of blocking inflammatory molecules such as the tumor necrosis factor alpha and more recently the axis that includes the interleukines 17 and 23, have allowed a sizeable improvement in the stratification of patients and in their clinical management.
Inspite of these advances answers are lacking on why spondylarthropathies commence, why the inflammatory response turns chronic and what determines the characteristics of the inflamed tissues. Absent an understanding of what causes and maintains these diseases, it is improbable that answers may be found to the fundamental questions of how to heal them. These questions often remain open in rheumatology, a discipline in which we are limited by our own ignorance about the causes of the morbous processes. Nevertheless, for no other condition is the feeling so precise that the pieces of the mosaic are available and that the problem consists in placing them. Solving the mistery of the spondylarthropathies could be the Trojan horse that would finally allow us to pierce the logic of rheumatic diseases and to heal them.
The Foundation has focused, from the moment when it began supporting the research on spondylarthropathies, on three burning questions:
- Why HLA-B*27.
- Why the pathogens.
- Why the entheses.
HLA-B*27 is a multimolecular molecular unit to which belong a heavy chain, beta2 microglobulin and peptides derived from the fragmentation of proteins. Expressing the unit is a nearly necessary condition to develop ankylosing spondylitis and a determining risk factor for other spondylarthropathies. However, only a fraction of the subjects who express the unit become ill. Thanks to the Foundation's support, Andreas Ziegler carried on, with his collaborators, biochemical, biophysical and biomolecular studies showing that the HLA-B*27 unit is endowed with a particular flexibility, a factor that may justify its instability and facilitate the activation of the cells of the immune system (see also below). The very recent identification of the effect of heavy metals on the structural characteristics and on the flexibility of HLA-B*27 in particular is an element of great importance in the definition of how environmental factors may affect the genetic background, creating the conditions that cause the disease to develop.> >
- identification of the biophysical characteristics that make HLA-B*27 unique.
Pathogens. As stated, HLA-B*27 is a unit in which peptidic protein fragments are an integrting part. In addition to HLA-B*27 as such, genes coding for the aminopeptidases ERAP1 and 2 enzymes involved in the generation of peptides also contribute to the risk of illness. The group led by Rosa Sorrentino and Mariateresa Fiorillo concentrated with the support of the Foundation on how HLA-B*27 and the associated peptides activate the key cells of the acquired immune system, the T lymphocytes. The results show that the structural characteristics of the HLA-B*27 unit, conditioned by the activity of ERAP1 and ERAP2, make it more effective than other histocompatibility molecules in terms of initiating responses against viruses that are common in the environment, such as the Epstein Barr virus; and that on the other hand the characteristics of susceptibility/resistance to spondylarthropathies may have been influenced over the course of generations by the presence of other environmental pathogens, such as the plasmodia that cause malaria (Plasmodium malariae).> >
- Peptides associated with HLA-B*27 activate T-lymphocytes
- autoreactive
- that recognise pathogens
Entheses. The autoimmune response caused by the recognition of the HLA-B*27/peptide unit is a systemic response, thus capable of involving the entire organism as a whole. However, the lymphocytes activated in the patients preferentially infiltrate the entheses, or similar structures from a molecular point of view, involved in the management of the stress associated with muscular contraction. Various factors that include the mechanical load and the production of cytokines such as IL-23 would lead> >
- to the activation of innate lymphocytes residing in the entheses
- to the production of signals capable of recalling other inflammatory cells.
In patients with spondylarthropathies this phenomenon would lead to the recall at enthese-level of self-reactive T-lymphocytes activated by the recognition of the HLA-B*27/peptide unit and to the generation of a vicious circle whereby the inflammatory response maintains and amplifies itself. The lymphocytes activated by the recognition of HLA-B*27 arrive preferentially at the hematopoietic marrow adjacent to the entheses and migrate through the expanded cortical vessels, causing over time the erosion of the tissue and the apposition of newly formed bone. Mariagrazia Uguccioni and her collaborators have in these years with the support of the Foundation initiated and carried on a vast dedicated project for the study of the lymphocytes of patients with spondylarthropathies and of the signals enabling them to migrate selectively, defining from the unit between a classical chemokine signal and HMGB1, an intracellular protein which is the prototype of the signals that cause inflammation in damaged tissues (Damage-Associated Molecular Patterns, DAMPs or alarmins).
Precision medicine. Only in a fraction of the patients who develop an osteoarticular response does the process chronicize, leading to illness. The identification of the mechanisms leading to the resolution or else to the chronicization of the inflammation, and which do or do not inform the development of chronic enthesitis and of erosion and remodeling of the bone, would have an irreplaceable value for purposes of putting into the proper biological context the molecular information we are obtaining from the molecular and functional studies summarized above. The group headed by Costantino Pitzalis has initiated and coordinated in the last decades an extraordinary effort to gather biological material from patients who show up with undifferentiated precocious arthritis in a tissue bank. With the support of the Foundation, Pitzalis' group initiated a study on these tissues, comparing the characteristics of those that do or do not evolve toward a spondylarthropathy and verifying the possibility that the distinctive elements of the tissue could predict the course of the disease and the response to the therapies.> >
- The tissues from patients who will develop a spondylarthropathy are a key to understanding how the illness mechanisms described above interact in the individual patient.
I am very pleased to point out the productivity of these lines of work, which can be clearly evaluated on the basis of the works published in prestigious international reviews in these few years, while a great many results are still to be published or in the course of being processed, especially for projects initiated more recently. The publication of the scientific data is not an end unto itself, but it is important because it allows to make the results available to the international scientific community, thus making them available without barriers for further advancements in our understanding of the disease that will be accomplished by other groups and laboratories.
Scientific works published thus far in international reviews surveyed by data banks with the support of the Ceschina Foundation:> >
- Cauli A, Dessole G, Piga M, Angioni MM, Pinna S, Floris A, Congia M, Mascia E, Paladini F, Tedeschi V, Sorrentino R, Fiorillo MT, Mathieu A. Expression analysis of HLA-E and NKG2A and NKG2C receptors points at a role for natural killer function in ankylosing spondylitis. RMD Open. 2018 Jul 13;4(2):e000597. doi: 10.1136/rmdopen-2017-000597.
- Cecchinato V, D'Agostino G, Raeli L, Nerviani A, Schiraldi M, Danelon G, Manzo A, Thelen M, Ciurea A, Bianchi ME, Rubartelli A, Pitzalis C, Uguccioni M. Redox-Mediated Mechanisms Fuel Monocyte Responses to CXCL12/HMGB1 in Active Rheumatoid Arthritis. Front Immunol. 2018 Sep 19;9:2118. doi: 10.3389/fimmu.2018.02118.
- Driller R, Ballaschk M, Schmieder P, Uchanska-Ziegler B, Ziegler A, Loll B. Metal-triggered conformational reorientation of a self-peptide bound to a disease-associated HLA-B*27 subtype. J Biol Chem. 2019 Jul 11. pii: jbc.RA119.008937. doi: 10.1074/jbc.RA119.008937
- Fiorillo MT, Paladini F, Tedeschi V, Sorrentino R. HLA Class I or Class II and Disease Association: Catch the Difference If You Can. Front Immunol. 2017, 8:1475.
- Loll B, Fabian H, Huser H, Hee CS, Ziegler A, Uchanska-Ziegler B, Ziegler A. Increased Conformational Flexibility of HLA-B*27 Subtypes Associated With Ankylosing Spondylitis. Arthritis Rheumatol. 2016 May;68(5):1172-82. doi: 10.1002/art.39567.
- Paladini F, Fiorillo MT, Vitulano C, Tedeschi V, Piga M, Cauli A, Mathieu A, Sorrentino R. An allelic variant in the intergenic region between ERAP1 and ERAP2 correlates with an inverse expression of the two genes. Sci Rep. 2018, 8(1):10398.
- Paladini F, Fiorillo MT, Tedeschi V, D'Otolo V, Piga M, Cauli A, Mathieu A, Sorrentino R: An ERAP2 promoter variant protects from ankylosing spondylitis in Sardinia and affects the expression of the HLA-B27 molecules. Rheumatology 2019: in press
- Paladini F, Fiorillo MT, Tedeschi V, Cauli A., Mathieu A, Sorrentino R: Ankylosing Spondylitis: a trade off of HLA-B27, ERAP and pathogen interconnections? Focus on Sardinia. Front Immunol. 2019 10:35.
- Tedeschi V, Vitulano C, Cauli A, Paladini F, Piga M, Mathieu A, Sorrentino R, Fiorillo MT. The Ankylosing Spondylitis-associated HA-B*2705 presents a B*0702-restricted EBV epitope and sustains the clonal amplification of cytotoxic T cells in patients Mol Med. 2016 Sep;22:215-223. doi: 10.2119/molmed.2016.00031.
- Tedeschi V, Alba J, Paladini F, Paroli M, Cauli A, Mathieu A, Sorrentino R, D'Abramo M, Fiorillo MT. Unusual Placement of an EBV Epitope into the Groove of the Ankylosing Spondylitis-Associated HLA-B27 Allele Allows CD8+ T Cell Activation. Cells. 2019 Jun 11;8(6). pii: E572. doi: 10.3390/cells8060572.
- Vitulano C, Tedeschi V, Paladini F, Sorrentino R, Fiorillo MT. The interplay between HLA-B27 and ERAP1/ERAP2 aminopeptidases: from anti-viral protection to spondyloarthritis. Clin Exp Immunol. 2017, 190:281-290.
- Yair-Sabag S, Tedeschi V, Vitulano C, Barnea E, Glaser F, Melamed Kadosh D, Taurog JD, Fiorillo MT, Sorrentino R, Admon A. The Peptide Repertoire of HLA-B27 may include Ligands with Lysine at P2 Anchor Position. Proteomics. 2018 May;18(9):e1700249. doi: 10.1002/pmic.201700249.
 I would also call to mind, within the framework of the initiatives launched in support of scientific research in these years, the beautiful international convention organized by Mariagrazia Uguccioni on the pathogenesis and mechanisms of spondylarthropathies in Lugano two years ago. The initiative garnered great success and on the basis of the discussions that took place we are planning a second international encounter, pursuant to similar modalities, to be held indicatively in the late Summer of 2021.
I would also call to mind, within the framework of the initiatives launched in support of scientific research in these years, the beautiful international convention organized by Mariagrazia Uguccioni on the pathogenesis and mechanisms of spondylarthropathies in Lugano two years ago. The initiative garnered great success and on the basis of the discussions that took place we are planning a second international encounter, pursuant to similar modalities, to be held indicatively in the late Summer of 2021.I am also pleased to announce the recent activation of the CESCHINA FOUNDATION INTERNATIONAL TRAINING FELLOWSHIP IN RHEUMATOLOGY program which runs under the supervision of Costantino Pitzalis. These scholarships are intended to complete the training of current or future specialists in rheumatology and related disciplines. The first Ceschina Foundation International Fellow, Dr. Federica Macaluso, has already begun
her training stint at the William Harvey Research Institute in London.
The investment in new generations of researchers and physicians is crucial for transmitting the intellectual passion toward the study of rare diseases, which is the Foundation's center of activity. It is this passion, guided by tenacity and intelligence, that will in the coming years radically change our way of studying and - hopefully healing patients affected by spondylartropathies.>
Projects
Project 1 (Germany) Research Group Prof. Andreas Ziegler
The human major histocompatibility complex is a genetic region that is of crucial importance for the immune system, the body's defence line against foreign invaders such as bacteria or viruses. It encodes several membrane proteins (HLA molecules) whose function it is to bind components (so-called peptides) derived from proteins of infectious agents that are present within cells. The resulting complexes can then be recognized by specialized white blood cells, so-called cytotoxic lymphocytes (CTL), and the cells carrying the complexes are destroyed as a consequence. In this way, the immune system can get rid of dangerous intruders. Unfortunately, this sophisticated ...
Project GIAS Università La Sapienza Roma (Italy) Research Group Prof.ssa Rosa Sorrentino
GIAS= Genetics and Immunobiology of Ankylosing Spondylitis
Rationale:
Ankylosing Spondylitis (AS) is a chronic, immune-mediated rheumatic disease, representing the prototypic member of a group of disorders known as Spondyloarthropathies. Patients with AS share clinical features such as spinal and pelvic joint dysfunction as well as genetic associations, primarily with genes involved in the antigen processing and presentation: the Human Leukocyte Antigen B*27 (HLA-B*27) and the Endoplasmic Reticulum Amino Peptidase 1 (ERAP1). In spite of the intense investigations, the AS pathogenic molecular mechanisms are still poorly elucidated. Noteworthy, the HLA-B*27 includes a family of more than one hundred ...
Project ASCHEM Institute for Research in Biomedicine Bellinzona (Switzerland) Research Group Dr. Mariagrazia Uguccioni
The group headed by Dr. Mariagrazia Uguccioni has described that the inflammatory milieu can dramatically change the response of leukocytes to chemokines. Among the many studies focused on human pathology, they have discovered that: i) inflammation results in an impairment of leukocyte cytoskeleton machinery that can be reverted by pharmacological intervention; ii) monocytes from patients with autoimmune diseases contribute to maintain the activity of the CXCL12/HMGB1 heterocomplex, fuelling inflammation, and iii) anti-chemokines antibodies, targeting an important region for receptor recognition, are present in patients with Ankylosing Spondylitis.
The project supported by the Ceschina Foundation analyse the ...
Project DISSECT-SPA Queen Mary University of London (UK) Research group Prof. Costantino Pitzalis
Discovering Novel Pathogenetic Mechanisms to Develop Effective Therapeutic Strategies
Queen Mary University of London (QMUL) www.qmul.ac.uk. QMUL is one of the world-class research-intensive higher-education institutions, part of the Russell Group, ranked 7th among multi-faculty universities at the most recent UK national assessment– the Research Excellence Framework (REF 2021).
QMUL offers cutting-edge research facilities and resources across the University, as well as many opportunities for interdisciplinary collaboration. QMUL, in partnership with ...
Project SPINET (Germany)
Neutrophil extracellular traps (NETS) orchestrate disease progression in patients with spondyloarthritis or diffuse idiopathic skeletal hyperostosis (DISH).
Research group:
Dr. Annika Heuer, University Medical Center Hamburg-Eppendorf
Prof. Michael Boettcher, University Medical Center Mannheim, University of Heidelberg
Dr. Jasmin Knopf, University Medical Center Mannheim, University of Heidelberg
Prof. Martin Herrmann, University Hospital Erlangen
Axial spondylarthritis (axSpA) and diffuse idiopathic skeletal hyperostosis (DISH) are prevalent conditions with serious clinical implications. The term axSPA describes inflammatory arthritis affecting the spine and/or the sacroiliac joints. It leads to variable ...
Notable result 2024
The group of Prof. Mariagrazia Uguccioni has published on February 23rd 2024 a study in RDM Open, Rheumatic and Musculoskeletal Diseases, describing a subpopulation of CD8+ T cells that might promote new bone formation and contribute to the pathological ossification process observed in Ankylosing Spondylitis. This study was possible thanks also to the support of the Fondazione Ceschina.
Patients with ankylosing spondylitis present a distinct CD8 T cell subset with osteogenic and cytotoxic potential
Veronica Martini#, Ylenia Silvestri#, Adrian Ciurea, Burkhard Möller, Gabriela Danelon, Flavio Flamigni, David Jarrossay, Ivo Kwee, Mathilde Foglierini, Andrea Rinaldi, Valentina Cecchinato§ and Mariagrazia Uguccioni§
# these are joint first authors
§ these are joint senior authors
Ankylosing spondylitis (AS) is a chronic inflammatory condition mainly affecting the axial skeleton and resulting in local new bone formation. Local inflammation in the spine, together with a mechanical stress, drive the release of several proinflammatory cytokines and chemokines, leading to an influx of immune cells, including CD8+ T cells. However, little is known on how the recruited immune cells and the chemokine system contribute to local bone deposition.
Thanks to the collaboration with the Rheumatology departments of the University of Zurich and Bern (Switzerland), the group led by Prof. Uguccioni has isolated CD8+CCR4+ T cells from the blood of patients with AS or healthy donors and analyzed them by multiparameter flow cytometry and gene expression sequencing.
CD8+CCR4+ T cells display a distinct effector phenotype and upregulate inflammatory chemokine receptors indicating an altered migration ability. In addition, CD8+CCR4+ T cells expressing the chemokine receptor CX3CR1 present an enhanced cytotoxic profile, and those from patients with active disease upregulate genes promoting osteogenesis, a core process in AS pathogenesis.
These findings shed light on a new molecular mechanism by which T cells may selectively migrate to inflammatory loci, promote new bone formation and contribute to the pathological ossification process observed in AS. A better understanding of the role of CD8+CCR4+ T cells and of the relevant chemokines promoting their migration to the bone, might provide a rationale for the development of additional novel treatments for AS.
Notable result 2023-3
In the first study, Tedeschi and co-workers showed that the AS-predisposing HLA-B*2705 allele can present an atypical EBV epitope which, by adopting an unusual binding groove placement, is able to evoke a vigorous CD8+ T cell response. This reactivity has been found in more than 70% of B*2705 positive carriers, mostly patients with AS. Such a behaviour is not shared by the non-AS-associated HLA-B*2709 molecule. This work sustains the concept of the mis-peptidome presented by the AS-risk factor B*2705 which can have implications in the disease chronic inflammation.
In the second study, the authors described how the HLA-B*2705-restricted CD8+ T cell responses against these suboptimal viral peptides and cross-reactive to self-peptides are influenced by the ERAP1 and ERAP2 haplotypes proving the cooperation between these AS risk factors in eliciting the adaptive immune responses in physiological and pathological conditions.
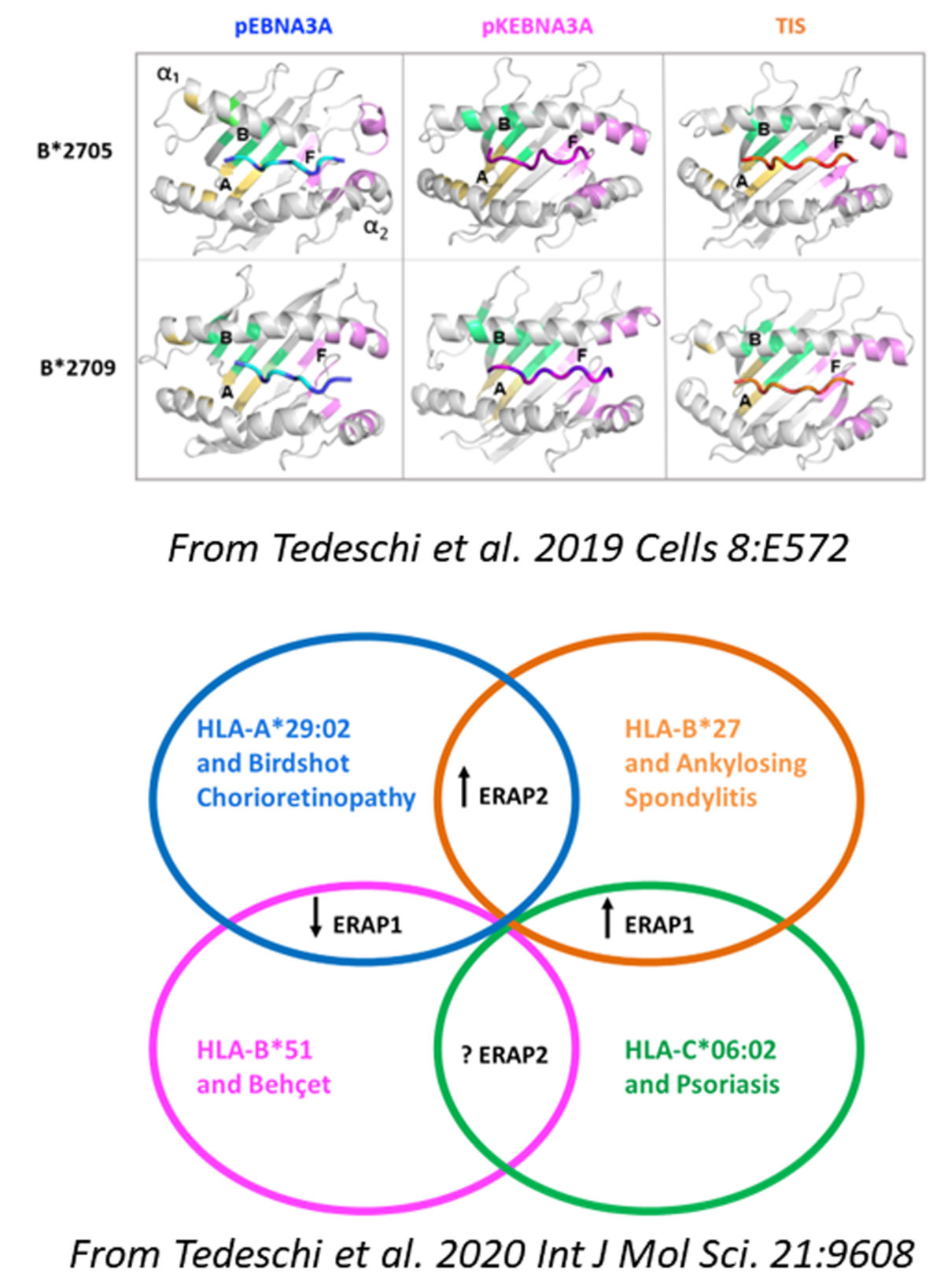
References:
Tedeschi V, Alba J, Paladini F, Paroli M, Cauli A, Mathieu A, Sorrentino R, D'Abramo M, Fiorillo MT. 2019. Unusual Placement of an EBV Epitope into the Groove of the Ankylosing Spondylitis-Associated HLA-B27 Allele Allows CD8+ T Cell Activation. Cells. 8: E572. DOI: 10.3390/cells8060572
Tedeschi V, Paldino G, Alba J, Molteni E, Paladini F, Scrivo R, Congia M, Cauli A, Caccavale R, Paroli M, Di Franco M, Tuosto L, Sorrentino R, D'Abramo M, Fiorillo MT. 2023. ERAP1 and ERAP2 Haplotypes Influence Suboptimal HLAB* 27:05-Restricted Anti-Viral CD8+ T Cell Responses Cross-Reactive to Self-Epitopes. Int J Mol Sci. 24:13335. DOI: 10.3390/ijms241713335
Related reviews:
Tedeschi V, Paldino G, Paladini F, Mattorre B, Tuosto L, Sorrentino R, Fiorillo MT. 2020. The impact of the ‘mispeptidome’ on HLA class I- mediated diseases: contribution of ERAP1 and ERAP2 and effects on the immune response. Int J Mol Sci. 21:9608. DOI: 10.3390/ijms21249608
Tedeschi V, Paldino G, Kunkl M, Paroli M, Sorrentino R, Tuosto L, Fiorillo MT. 2022. CD8+ T Cell Senescence: Lights and Shadows in Viral Infections, Autoimmune Disorders and Cancer. Int J Mol Sci. 23:3374. DOI: 10.3390/ijms23063374
Notable result 2023-2
The study by Mattorre et al. has described for the first time a "short form" of ERAP2 which is expressed in macrophages independently from gene variance. It is generated by autocatalytic cleavage in an acidic environment, cooperates with LNPEP/IRAP and acts as regulator of the Renin-Angiotensin System extending its role beyond the classical antigen processing function.
The "hypothesis and theory" article by Paladini et al. carries out a comprehensive evolutionary analysis of ERAP1, ERAP2 and LNPEP/IRAP aminopeptidases considering their multi-faceted activities and expression along the zoological scale. This has allowed to hypothesize gene duplication events from the progenitor LNPEP/IRAP by which ERAP1 and ERAP2 have been derived and the acquisition of antigen processing functions as secondary to their first role as regulators of vascular response through the modulation of Renin-Angiotensin System. Given the implications in the inflammatory process, this finding opens a novel and additional view of the ERAP2 involvement in the MHC-I-associated diseases among which psoriasis and AS. In particular, the risk conferred by ERAP2 in AS appears to be independent of the HLA-B*27, suggesting that the process by which ERAP2 affects the disease pathogenesis could be different from antigen processing.
These preliminary observations deserve further investigations.
References:
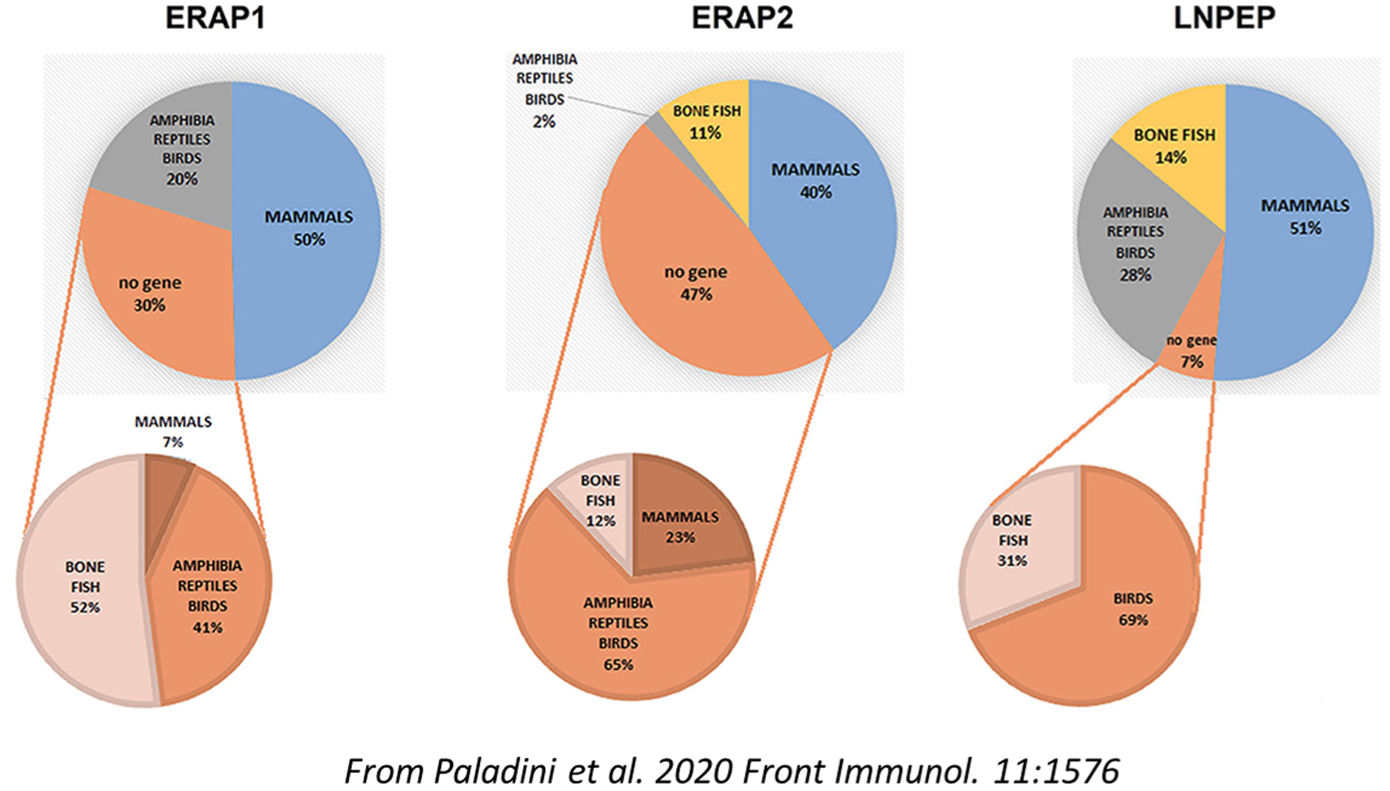 Mattorre B, Caristi S, Donato S, Volpe E, Faiella M, Paiardini A, Sorrentino R, Paladini F. 2022. A Short ERAP2 That Binds IRAP Is Expressed in Macrophages Independently of Gene Variation. Int J Mol Sci. 23:4961. DOI: 10.3390/ijms23094961
Mattorre B, Caristi S, Donato S, Volpe E, Faiella M, Paiardini A, Sorrentino R, Paladini F. 2022. A Short ERAP2 That Binds IRAP Is Expressed in Macrophages Independently of Gene Variation. Int J Mol Sci. 23:4961. DOI: 10.3390/ijms23094961Mattorre B, Tedeschi V, Paldino G, Fiorillo MT, Paladini F, Sorrentino R. 2022. The emerging multifunctional roles of ERAP1, ERAP2 and IRAP between antigen processing and renin-angiotensin system modulation. Front Immunol. 13:1002375. DOI: 10.3389/fimmu.2022.1002375
Paladini F, Fiorillo MT, Tedeschi V, Mattorre B, Sorrentino R. 2020. The multifaceted nature of aminopeptidases ERAP1, ERAP2 and LNPEP: from evolution to disease. Front Immunol. 11:1576. DOI: 10.3389/fimmu.2020.01576
Notable result 2023-1
The group of Prof. Mariagrazia Uguccioni, together with the group of Prof. Robbiani has recently published a study in Nature Immunology on March 6th, also thanks to the support of the Fondazione Ceschina.
Autoantibodies against chemokines post-SARS-CoV-2 infection correlate with disease course
Jonathan Muri#, Valentina Cecchinato#, Andrea Cavalli, Akanksha A Shanbhag, Milos Matkovic, Maira Biggiogero, Pier Andrea Maida, Jacques Moritz, Chiara Toscano, Elaheh Ghovehoud, Raffaello Furlan, Franca Barbic, Antonio Voza, Guendalina De Nadai, Carlo Cervia, Yves Zurbuchen, Patrick Taeschler, Lilly A Murray, Gabriela Danelon-Sargenti, Simone Moro, Tao Gong, Pietro Piffaretti, Filippo Bianchini, Virginia Crivelli, Lucie Podešvová, Mattia Pedotti, David Jarrossay, Jacopo Sgrignani, Sylvia Thelen, Mario Uhr, Enos Bernasconi, Andri Rauch, Antonio Manzo, Adrian Ciurea, Marco B L Rocchi, Luca Varani, Bernhard Moser, Barbara Bottazzi, Marcus Thelen, Brian A Fallon, Onur Boyman, Alberto Mantovani, Christian Garzoni, Alessandra Franzetti-Pellanda, Mariagrazia Uguccioni§, Davide F Robbiani§
# these authors contributed equally
§ these authors jointly supervised this work
See also News & Views and In Brief:
News & Views: The kinetics of chemokine autoantibodies in COVID-19
Furong Qi, Dapeng Li & Zheng Zhang
Nature Immunology, March 15th, 2023
In Brief: Autoantibodies against chemokines linked with better disease outcomes in COVID-19
Ivonne Bordon
Nature Review in Immunology, 23:203, 2023
The study has discovered that autoantibodies anti-chemokines that may arise after COVID-19 infection are able to neutralize the activity of these molecules, which are essential to direct immune cell are trafficking. In the presence of the autoantibodies, the traffic of leukocytes is impaired, and this might result in turning off the inflammatory response.
But how does blocking the immune response help in COVID-19? The immune system in some cases is a double-edged sword, it is important to activate it promptly to neutralize the coronavirus, but it must also be turned off at the right time, otherwise the continuous inflammation might result in tissue damage. In fact, what usually brings patients to the hospital is excessive inflammation induced by the infection. Therefore, autoantibodies against chemokines could have a beneficial anti-inflammatory effect.
The researcher have discovered that not only individuals recovered from COVID-19 develop anti-chemokine antibodies, but that this type of antibodies are present also in patients with Ankylosing Spondylitis and other autoimmune diseases. The research is now focused on understanding if these antibodies are associated to various disease trajectories, and how their presence is beneficial for dampening chronic inflammation.
Notable result 2019
"Ankylosing Spondylitis and Related Immune-Mediated Disorders" published in Frontiers in Immunology. This research topic has gathered a series of articles (Reviews, Mini Reviews, Perspective and Hypothesis and Theory articles) providing a comprehensive overview of basic and translational on-going research in the field of the disease. Frontiers in Immunology is an open access journal. These useful resources are available as such for the scientific community and for interested reader via the link above.
Notable result 2018-2019
Genetic susceptibility to ankylosing spondylitis is due to several genes with the HLA-B27 playing a major role. HLA-B27 is involved in the antigen presentation. Other genes impacting on the selection of the antigenic repertoire contribute to the disease. Among these the ERAPs, which shape the HLA immunopeptidome. In humans, there are two contiguous ERAP1 and ERAP2 genes, both involved in AS pathogenesis. However, while ERAP1 and HLA-B27 are epistatic in conferring AS susceptibility, probably because ERAP1 generates a predisposing peptide repertoire, ERAP2 plays an independent and less defined role. ERAP2 displays a balanced polymorphism resulting in the presence or absence of the enzyme. Its absence is protective in ankylosing spondylitis and other inflammatory diseases.
In the first study, the authors report a genetic variation that maps in the ERAP2 promoter. This variation determines an inverse balance in the expression of the two ERAPs. In the presence of the minor allele, possessed by a small percentage of the population (7-15%), ERAP2 expression is reduced and ERAP1 is increased whereas the opposite occurs in the presence of the major allele. This is the first demonstration that the two genes are transcriptionally interconnected.
The second study has been performed in Sardinia, where the minor allele is present at higher frequency than in other populations. Results highlight a protective role for this variation. The expression of HLA-B*2709, an HLA variant described by the authors not to be associated with ankylosing spondylitis, is more dependent on the polymorphism in comparison to the disease-associated HLA-B*2705. Thus the minor allele correlates with a lower expression of HLA-B*2709, indicating that the balance between ERAP1 and ERAP2 is relevant for the shaping of a peptidome suitable for the HLA-B*2709 molecules. In conclusion, the data show that in Sardinia there is a higher frequency of genetic variants (HLA-B*2709; ERAP2) with a protective effect on AS and that, combined, decrease the overall expression of the HLA-B27 molecules. In Sardinia the susceptibility variant maps in ERAP2 promoter region and is inherited as a quantitative trait suggesting that the fine-tuning of the two ERAPs is relevant for disease pathogenesis. To explain this unusual combination of events, the authors suggest that malaria, endemic in the Mediterranean island, has contributed to shape a genetic framework that has become protective to AS.
Why these results are important:
HLA-B27 is a major player in the pathogenesis of ankylosing spondylitis. Two proteolytic enzymes, ERAP1 and ERAP2 also contribute to the risk of the disease, but the mechanisms are elusive. These results indicate that:
- the relative expression of the two genes is finely regulated
- the balance impacts on the expression of HLA-B27 molecules.
This regulation has perhaps offered advantages in areas such as Sardinia to protect the population from malaria and, in turn, could be involved in determining protection/susceptibility to the ankylosing spondylitis.
References
Paladini F, Fiorillo MT, Vitulano C, Tedeschi V, Piga M, Cauli A, Mathieu A, Sorrentino R. An allelic variant in the intergenic region between ERAP1 and ERAP2 correlates with an inverse expression of the two genes. Sci Rep. 2018, 8(1):10398.
Paladini F, Fiorillo MT, Tedeschi V, D’Otolo V, Piga M, Cauli A, Mathieu A, Sorrentino R: An ERAP2 promoter variant protects from ankylosing spondylitis in Sardinia and affects the expression of the HLA-B27 molecules. Rheumatology 2019: doi: 10.1093/rheumatology/kez212, in press
Associated reviews and comments:
Vitulano C, Tedeschi V, Paladini F, Sorrentino R, Fiorillo MT. The interplay between HLA-B27 and ERAP1/ERAP2 aminopeptidases: from anti-viral protection to spondyloarthritis. Clin Exp Immunol. 2017, 190:281-290.
Fiorillo MT, Paladini F, Tedeschi V, Sorrentino R. HLA Class I or Class II and Disease Association: Catch the Difference If You Can. Front Immunol. 2017, 8:1475.
Paladini F, Fiorillo MT, Tedeschi V, Cauli A., Mathieu A, Sorrentino R: Ankylosing Spondylitis: a trade off of HLA-B27, ERAP and pathogen interconnections? Focus on Sardinia. Front Immunol 2019 10:35.
Notable result 2016-2
The Ankylosing Spondylitis-associated HLA-B*2705 presents a B*0702-restricted EBV epitope and sustains the clonal amplification of cytotoxic T cells in patients.
Tedeschi V, Vitulano C, Cauli A, Paladini F, Piga M, Mathieu A, Sorrentino R, Fiorillo MT.
Mol Med. 2016 May 18;22. doi: 10.2119/molmed.2016.00031. [Epub ahead of print] PMID: 27254288 Free PMC Article
The study explores in detail the ability of HLA-B27 variants associated or not with the susceptibility to spondylarthropathies to present fragments derived from the processing of viral proteins to T lymphocytes. Specifically they report for the first time that the disease-associated HLA-B*2705 evokes in more than 60% of HLA-B27-positive subjects a vigorous response of CD8+ T cells in the presence of a definedsequence derived from the Epstein-Barr virus (EBV). EBV is a human herpes virus that infects >90% of individuals. The infection is often subclinical, a condition that reflects the ability of the virus to preserve the host on the one hand and on the other the existence of effective and refined mechanisms through which the immune system of the host maintains viral latency – for an excellent recent review, we refer the interested reader to Tangye SG & al. Human immunity against EBV-lessons from the clinic.
J Exp Med. 2017 Feb;214(2):269-283. doi: 10.1084/jem.20161846. The viral peptide studied by Tedeschi and coll. is a known immunodominant sequence presented in the context of a different HLA molecule (HLA-B7) and per se lacks a classical B27 consensus motif. Of importance the peptide cannot be presented by the non-disease-associated HLA-B*2709 molecules neither HLA-B*2709 positive individuals possess CD8+ T cells specific for this peptide. Overall, this study indicates that the higher plasticity of the HLA-B*2705 groove might link the protection against common virus with the susceptibility to autoimmunity, a trade-off that has never been so far ever dissected so finey at the molecular level.
Notable result 2016-1
The group of Prof. Ziegler has recently published on the well-respected international journal Arthritis & Rheumatology, also thanks to the support of the Fondazione Ceschina, the study
Loll B, Fabian H, Huser H, Hee CS, Ziegler A, Uchanska-Ziegler B, Ziegler A.
Arthritis Rheumatol. 2016 May;68(5):1172-82. doi: 10.1002/art.39567.
Increased Conformational Flexibility of HLA-B*27 Subtypes Associated With Ankylosing Spondylitis.
See also editorial comment:
Editorial: HLA-B27: The Story Continues to Unfold.
Powis SJ, Colbert RA.
Arthritis Rheumatol. 2016 May;68(5):1057-9. doi: 10.1002/art.39566
The study deals with the molecular basis of the association between HLA–B27 and spondyloarthritis, ankylosing spondylitis (AS) in particular. Despite intense efforts in the last four decades the reasons of the link remain unknown. The results of the group of Andreas Ziegler adds to our understanding of the peculiar features of HLA-B27, which has unique folding and unfolding properties, thus yielding free heavy chains and aberrant complexes on the plasma membranes. Specifically, the authors compare results obtained on HLA-B27 variants that are associated or not to the disease and infrared spectroscopy, that allows to explore the dynamic properties of proteins. They observe that conformational flexibility of the disease associated HLA-B27 is greater. This feature might be crucial for the pathogenesis of AS, possibly accounting for increased heavy chain dimerization or for ER stress and as proposed in the accompanying editorial for less effective negative selection of self-reactive CD8+ T cells and therefore easier autoreactivity. As Powis and Colbert comment:
Detailed and careful structural analyses such as those provided by Loll et al provide an important perspective on the biology of HLA–B27 variants that may prove to be relevant to disease pathogenesis. They emphasize the need for further studies probing structural differences, including dynamic processes such as folding and unfolding. Incorporating complex mixtures of peptides generated with varying influence of ERAP 1/2 that better mimic the ER environment is a challenge that, if met, may provide unprecedented insights into the role of HLA–B27 in spondyloarthritis.
Events 2023
2nd International Workshop on Ankylosing Spondylitis: tales of molecules and patients.
Institute for Research in Biomedicine
Bellinzona - Switzerland
Spondyloarthritis encompasses a group of inflammatory diseases characterized by shared clinical features and genetic markers, including ankylosing spondylitis, reactive arthritis, arthritis associated with conditions such as psoriasis or inflammatory bowel disease. The clinical landscape is dominated by the interplay of articular and extra-articular inflammation, encompassing enthesitis, dactylitis, uveitis, bone destruction, and new bone formation. While the association between the HLA-B27 MHC class I molecule and spondyloarthritis has long been recognized, the mechanisms through which genetics contributes to pathogenesis remain elusive. Recent research underscores the increasing significance of leukocytes from both the innate and acquired arms of the immune response in shaping the epithelial inflammatory response to sterile and microbial stimuli. This paradigm extends to the microbiome and its interaction with the gut immune system, supporting a system in which antimicrobial response, autoimmune reactions, and physiological responses to mechanical tissue damage are intricately intertwined. The poor understanding of the molecular hierarchy associated with spondylarthritis is evident in our failure to devise effective treatment strategies capable of not only addressing symptoms and improving facets of patients' quality of life but also breaking the vicious circle that sustain the condition and ultimately achieving healing. The mission of the Fondazione Ceschina is to encourage creative, out-of-the-box thinking that may lead to novel insights into solving the enigma of spondylarthritis. The contributions presented during this meeting in Bellinzona, organized by Prof. Uguccioni and her colleagues under the auspices of the Fondazione, provided a focused perspective on a rapidly evolving landscape where new knowledge is accumulated and integrated. The tales “of molecules and patients” shared by groups supported by the Fondazione, along with insights from exceptional host scientists, vividly portrayed a dynamic field where answers to longstanding questions are drawing closer through the diligent efforts of scientists, physicians, and patients. The meeting was held in the beautiful new building of the Institute for Research in Biomedicine (IB), which relocated to its new headquarters in Bellinzona in 2021. This served as a perfect and stimulating environment for both speakers and attendees, the majority of whom were young researchers eager to contribute to the dynamic discussions and collaborative spirit of the event.
It has been an especially gratifying experience to juxtapose the landscape that unfolded during the inaugural international meeting in Lugano in 2017, also organized under the auspices of the Fondazione Ceschina, with the insights gleaned from this recent gathering. The crucial stimuli presented in the first forum have not only been received but also meticulously elaborated upon with creativity, resulting in significant breakthroughs that extend far beyond the confines of the specific field. The progress achieved by the groups supported by the Fondazione Ceschina over these few years has indeed been substantial. The intellectual ferment generated by these interactions has not only propelled advancements within the targeted research domain but has also had far-reaching implications, illustrating the profound impact that collaborative efforts and innovative thinking can have on scientific inquiry.
Event Gallery
Events 2021
Due to the COVID-19 pandemic, the periodic meeting of the Ceschina Foundation was held virtually on June 17, 2021. The virtual meeting was a great success, with substantial progress being achieved thanks to the support of the Ceschina Foundation, despite the difficult and often dramatic conditions we have had to face in the last year. The image represents some of the participants.
Events 2019
Chia Laguna, Domus de Maria (Cagliari) - Italy
23 - 24 June, 2019
The Ceschina Foundation round table entitled "Work in progress on seronegative spondyloarthropathies" took place in June 2019 in the splendid scenery of Chia Laguna, Domus de Maria near Cagliary (Italy). The meeting is a forum for established and younger scientists who are working with the support of Ceschina Foundation in different areas of the mechanisms responsible for the initiation and maintenance of inflammation and maladaptive tissue remodeling in rare diseases, with emphasis on spondyloarthropathies. The seminar is usually held twice a year and offers a unique opportunity for a small group of scientists to communicate their work and discuss its future directions. The issues discussed in Chia Laguna included the state of the art on the work on HLA-B27 molecules and related gene products, on the cells of the immune system that are recruited and kept alive at the sites of inflammation, new suggestions for the pathogenesis of the disease from genetics and functional genomics and applications of precision medicine to dissect patient heterogeneity. The program allowed a plenty of time and energy for fruitful and enjoyable discussion. The frequent meetings organized by the Foundation have proved to be an effective tool to foster communication among researchers operating in nearby, even if not overlapping, fields optimizing the exchange of ideas and cross-contamination. The next periodic meeting is scheduled for December 2019 in Milano, Italy.
Events 2017
Auditorium - Università della Svizzera italiana, Lugano, Switzerland
September 30 - October 1, 2017
Spondyloarthritis comprises inflammatory diseases that share clinical features and genetic markers, such as ankylosing spondylitis, reactive arthritis, and arthritis associated with psoriasis or inflammatory bowel disease. Articular and extra-articular inflammation such as enthesitis, dactylitis and uveitis, bone destruction and new bone formation are only partially inter-connected and dominate the clinical scenario. The relationship between the HLA-B27 MHC class I molecule and spondyloarthritis has been known for over 40 years, but the mechanisms by which it contributes to the pathogenesis are still elusive. Research in recent years supports a role for T cells and phagocytes in determining epithelial antimicrobial responses, a paradigm that might apply to the microbiome and its interaction with the gut immune system. The factors that lead to the inexorable activation of inflammatory pathways and to the phenotype of the disease remain unknown. More generally, a comprehensive vision of the pathogenesis is lacking, probably due to the lack of integration of the information simultaneously generated in various fields. Indeed results crucial to identify the mechanisms of spondyloarthritis and as such to allow better treatments of the disease might perfectly derive from research on apparently unrelated topics Therefore, a main goal is to bring together scientists working on the various characteristics of spondyloarthritis and on related fields, creating the environment for cross-contamination between different approaches.
On September 30-October 1, 2017 the Fondazione Ceschina has supported the organization of the first International workshop on "Ankylosing Spondylitis: tales of molecules and patients" chaired by Maria Grazia Uguccioni (Bellinzona), which took place in the Università della Svizzera Italiana in Lugano, Switzerland. The program covered several new developments in academic research in the field, with the aim of defining the burning questions that should be addressed and the missing links that should be identified.
Why the enthesis? Costantino Pitzalis (London) opened the meeting, exposing the audience to the use of molecular and precision medicine tools towards the definition of a taxonomy of rheumatologic diseases. His results indicate how studying the synovial pathology of patients with early undifferentiated arthritis requires careful standardization (Humby F et al, 2018) and could represent a privileged system for early diagnosis and treatment, providing predictive suggestions on the response to targeted molecular therapies. Dennis McGonagle (Leeds) provided in the subsequent presentation a comprehensive overview of the state of the art on the prevalent inflammatory involvement of the enthuses, the insertions of ligaments and tendons to the bone. Enthesitis represents a major feature of spondyloarthritis, involves biomechanical and immunological events. Innate immune cells are involved, together with entheseal cells that respond to IL-23 by producing inflammatory signals such as TNFalpha, IL-17 and IL-22. This latter cytokine has a consolidated role in the regulation of stem cells in the skin and in the gut (El-Zayadi AA et al., 2017). The reported data convincingly suggest that IL-22 regulates the function of human mesenchymal stem cells in inflammatory environments, influencing osteogenesis and possibly contributing to physiological bone repair and to abnormal bone formation at the entheses in human spondyloarthritis.
Why the microbes? Subclinical intestinal inflammation is emerging as a frequent event and a relevant actor in the pathogenesis of spondyloarthritis. Maria Rescigno (Milan) focused on the evidence that a gut-vascular barrier controls in mammalians the translocation of microbial products and antigens into the bloodstream (Spadoni I et al., 2015). Bacteria such as Salmonella typhimurium which systemically disseminate penetrate the barrier by modulating the Wnt/β-catenin pathway into the epithelial cells. In contrast, disruption of intestinal barriers is associated with changes in activity and gene expression of innate immune cells at distant sites that cause seemingly unrelated inflammation in extra-intestinal tissues and organs. Francesco Ciccia (Palermo) discussed the alterations of the vascular and epithelial barriers in patients with spondyloarthritis. Dysbiosis in patients is frequent, correlates with the severity of entheseal inflammation and jeopardizes the integrity of gut-epithelial and gut-vascular barriers, eventually causing the translocation of microbial products in the blood (Ciccia et al., 2017). In these patients ileal microbes elicited the downregulation of the tight junction proteins, claudin 4 and occludin and conversely the upregulation of zonulin, which is associated to the permeability of gut epithelial cells tight junctions. IL-23, whose expression is regulated in the intestine of patients with spondyloarthritis via autophagy, contributes to the expansion of gut innate immune cells, which possibly recirculate to distant sites of active inflammation.
Microbiota, IgA and gut inflammation. Emma Wetter Slack (Zurich) focused on whether which mucosal IgA, which are crucial player in maintaining the homeostasis of the intestine, control the intestinal microbiome. She described a mechanism that includes in vivo the cross-linking and the enchainment of the daughter cells of rapidly dividing bacteria in the lumen of the gut, even at relatively low densities and without the requirement of inflammation or killing event. Enchained cells cannot separate after division, yielding clumps. By this mechanism the clearance of the microbes from the gut lumen is accelerated while plasmid-donor and plasmid -recipient cell clones remain separate, restricting plasmid transfer in vivo (Moor K et al, 2017). Intestinal microbiota represent a source of extracellular ATP, a well-established endogenous inflammatory signal, which is responsible of various features of the inflammatory response via purinergic receptors, such as the ATP-gated ionotropic P2X7 receptor This pathway is relevant, in particular considering that via P2X7 microbiota signals influence T follicular helper cells in the gut associated lymphoid tissue and eventually modulate the secretion of affinity-matured IgA and the interaction with commensal microbes (Perruzza L. et al, 2017). Fabio Grassi (Bellinzona) has discussed the role of the P2X7 receptor in the induction and the maintenance of autoimmune responses, which in turn influence the outcome of self-sustaining inflammatory processes. The intestinal network regulating IgA production is among the targets of biotechnological treatments in patients with spondylarthritis (Wang WB et al, 2017). Conversely some intestinal E. coli are preferentially coated by IgA in patients with spondylarthritis associated to Crohn's disease and this event is mechanistically linked to the expansion of TH17 lymphocytes (Viladomiu M et al, 2017), further highlighting the role of the interaction between the gut immune system and the microbiota in causing the disease.
Why the HLA-B27? Sebastian Springer (Bremen) had underlined the molecular constrains regulating the association of the human HLA-B*27:05 protein to spondylarthritis, finely analysing how the peptide binding site of the molecules appears per se disordered due to charge repulsions in the absence of peptide. Thus the molecule appears to be substantially more dependent than other HLA-B27 molecules less associated to the disease on chaperone protein for the overall stability of the complex. The availability of novel tools, including small molecules, that can influence peptide binding and MHC class I structural characteristics paves the way to intriguing biotechnological applications, in the spondylarthritis field.
HLA-B27 is ubiquitously expressed. However, spondylarthritis targets a relatively limited number of tissues and organs, such as the entheses, the gut and the spleen. Regulating the migratory capacities and arrest of leukocytes at these inflammatory sites depends on the chemokine system, which might contribute to shape the disease features in terms of preferential involvmenet of selected sites. CXCL12 is a protean signal that controls leukocyte migration and downstream immune responses on the one hand and on the other regulates progenitor cells migration and recruitment to sites of injury, influencing tissue repair, adipogenesis and osteogenesis (see above). HMGB1, the prototypic sterile endogenous inflammatory signal, works at least in part by forming heterocomplexes that enhance of the function of CXCL12. Andrea Cavalli (Bellinzona) discussed the structural characteristics of the CXCL12/HMGB1 complexes, that could represent a key to the self-sustaining loop between inflammation, bone destruction, unrelenting repair and novel bone formation in patient with spondylarthritis.
Beyond genetics. Gioacchino Natoli (Milan) focused on the mechanisms that influence at transcriptional and post-transcriptional levels the response of innate immune cells. The response to microbial components and to sterile signals “both involved in the pathogenesis of spondylarthritis as discussed by previous speakers“ is highly integrated and requires the involvement of various cell types with intrinsically distinct characteristics. Early responses are not confined to a single responding cell type and involve potentially responsive immune and parenchymal tissue cells. The functional specification of mature immune cells, the differentiation of which depends on the combinatorial activity of relatively few transcription factors with overlapping expression profiles, defines their response to endogenous inflammatory signals, such as extracellular ATP or HMGB1. A single inflammatory stimulus might elicit distinct functional outcomes in myeloid cells depending on a specific genomic platform for individual cell types. The complex scenario sketched in spondylarthritis, in which distant cells in various organs interact in a network, might represent a challenging environment to explore "the genomic landscapes" of chronic inflammation (Monticelli and Natoli, 2017).
Epigenomic characteristics have been widely profiled on cell types, tissues and individuals. Several correlations have been identified, but it is difficult to determine which signs are directly involved in gene regulation. Stephan Beck (London) has discussed the factors that influence DNA methylation, describing the evidence to support not only the influence of genetic and environmental variations between individuals, but how the impact on these changes in the environment increases with the age. Finally, Oreste Acuto (Oxford) focused on the positive selection of the T cell repertoire in the thymus, a process that allows weakly auto-reactive T cells to survive and to differentiate. The THEMIS protein has the same name as the ancient Greek Titaness, usually represented as holding a sword, useful for cutting facts from fiction. THEMIS (the protein, not the Titaness) is well expressed in conventional T cells but virtually absent in regulatory T cells and in subsets of intestinal lymphocytes. In developing thymocytes it forms with the tyrosine phosphatases SHP1 or SHP2 a complex that modulates T cell receptor phosphorylation and finely adjusts the survival, development and activation threshold of T cells. Premature aging of T cells in spondyloarthritis probably reflects the early contraction of the thymic output and the homeostatic proliferation of the remaining cells (Fessler J. et al., 2016). The molecular bases of the premature failure of the thymus and the possible involvement of signals that regulate the signaling downstream of T cell receptor activation in patients with these diseases remain to be investigated.
The meeting, in the evocative setting of Lake Lugano, was an occasion for lively discussions. Several outstanding questions remain, for example why is HLA-B27 involved in spondylarthritis, why are entheses the preferential site of inflammation, what is the role of recognition of microbial components and of alarmins, how inflammation and bone destruction/remodeling are interrelated. There remains a lot to do in addressing these questions. We anticipate huge progress in the coming years, which will help us to find a unique answer to the complexities of spondylarthritis.
References
Ciccia F, Guggino G, Rizzo 2, Alessandro R, Luchetti MM, Milling S, Saieva L, Cypers H, Stampone T, Di Benedetto P, Gabrielli A, Fasano A, Elewaut D, Triolo G. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis. 2017 Jun;76(6):1123-1132. doi: 10.1136/annrheumdis-2016-210000.
El-Zayadi AA, Jones EA, Churchman SM, Baboolal TG, Cuthbert RJ, El-Jawhari JJ, Badawy AM, Alase AA, El-Sherbiny YM, McGonagle D. Interleukin-22 drives the proliferation, migration and osteogenic differentiation of mesenchymal stem cells: a novel cytokine that could contribute to new bone formation in spondyloarthropathies. Rheumatology (Oxford). 2017 Mar 1;56(3):488-493.
Fessler J, Raicht A, Husic R, Ficjan A, Duftner C, Schwinger W, Dejaco C, Schirmer M. Premature senescence of T-cell subsets in axial spondyloarthritis Ann Rheum Dis. 2016 Apr;75(4):748-54. doi: 10.1136/annrheumdis-2014-206119.
Humby F, Romão VC, Manzo A, Filer A, Bugatti S, Vieira-Sousa E, Kelly S, Wechalekar M, Ahmed M, Rocher V, Hands R, Montecucco C, Fonseca J, Pitzalis C. A Multicenter Retrospective Analysis Evaluating Performance of Synovial Biopsy Techniques in Patients With Inflammatory Arthritis: Arthroscopic Versus Ultrasound-Guided Versus Blind Needle Biopsy.Arthritis Rheumatol. 2018 May;70(5):702-710. doi: 10.1002/art.40433.
Monticelli S, Natoli G. Transcriptional determination and functional specificity of myeloid cells: making sense of diversity. Nat Rev Immunol. 2017 Oct;17(10):595-607. doi: 10.1038/nri.2017.51.
Moor K, Diard M, Sellin ME, Felmy B, Wotzka SY, Toska A, Bakkeren E, Arnoldini M, Bansept F, Co AD, Völler T, Minola A, Fernandez-Rodriguez B, Agatic G, Barbieri S, Piccoli L, Casiraghi C, Corti D, Lanzavecchia A, Regoes RR, Loverdo C, Stocker R, Brumley DR, Hardt WD, Slack E. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature. 2017 Apr 27;544(7651):498-502. doi: 10.1038/nature22058.
Perruzza L, Gargari G, Proietti M, Fosso B, D'Erchia AM, Faliti CE, Rezzonico-Jost T, Scribano D, Mauri L, Colombo D, Pellegrini G, Moregola A, Mooser C, Pesole G, Nicoletti M, Norata GD, Geuking MB, McCoy KD, Guglielmetti S, Grassi F. T Follicular Helper Cells Promote a Beneficial Gut Ecosystem for Host Metabolic Homeostasis by Sensing Microbiota-Derived Extracellular ATP Cell Rep. 2017 Mar 14;18(11):2566-2575. doi: 10.1016/j.celrep.2017.02.061.
Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, Penna G, Dejana E, Rescigno M. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015 Nov 13;350(6262):830-4. doi: 10.1126/science.aad0135.
Viladomiu M, Kivolowitz C, Abdulhamid A, Dogan B, Victorio D, Castellanos JG, Woo V, Teng F, Tran NL, Sczesnak A, Chai C, Kim M, Diehl GE, Ajami NJ, Petrosino JF, Zhou XK, Schwartzman S, Mandl LA, Abramowitz M, Jacob V, Bosworth B, Steinlauf A, Scherl EJ, Wu HJ, Simpson KW, Longman RS. IgA-coated E. coli enriched in Crohn's disease spondyloarthritis promote TH17-dependent inflammation. Sci Transl Med. 2017;9(376). pii: eaaf9655. doi: 10.1126/scitranslmed.aaf9655.
Wang XB, Ellis JJ, Pennisi DJ, Song X, Batra J, Hollis K, Bradbury LA, Li Z2, Kenna TJ, Brown MA. Transcriptome analysis of ankylosing spondylitis patients before and after TNF-α inhibitor therapy reveals the pathways affected. Genes Immun. 2017 Sep;18(3):184-190. doi: 10.1038/gene.2017.19. Epub 2017 Aug 24.
Fondazione Ceschina
- C/O Altiqa SA
- Via Nassa 21
- 6900 Lugano
- Svizzera









